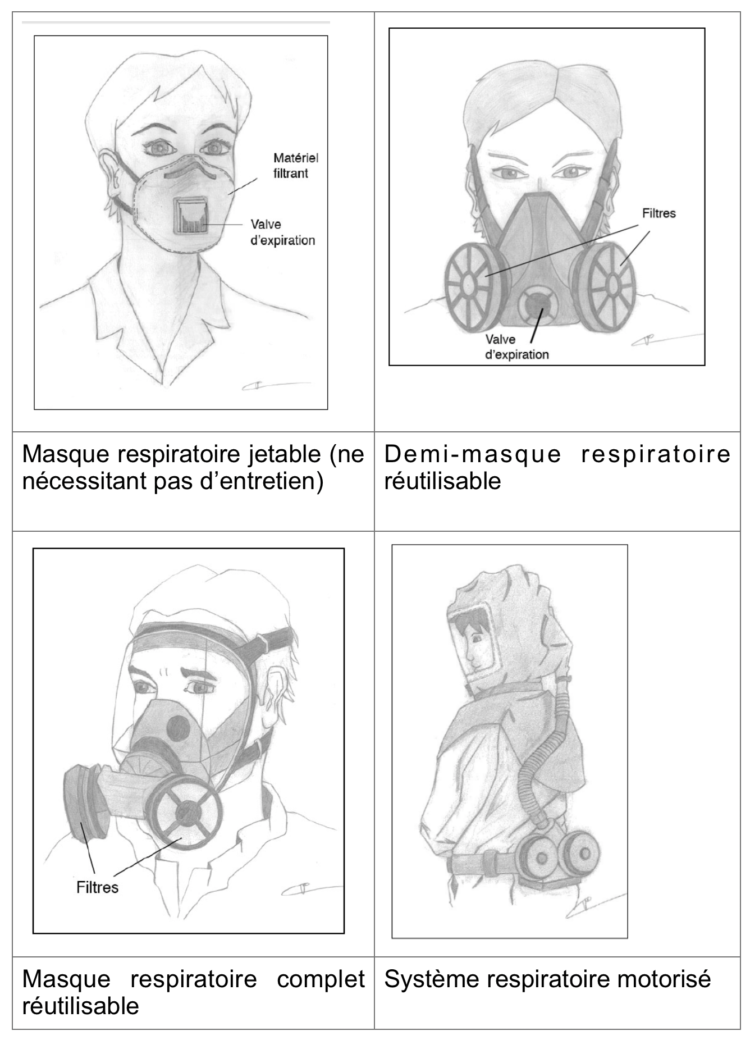
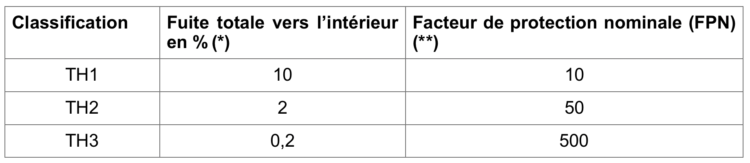
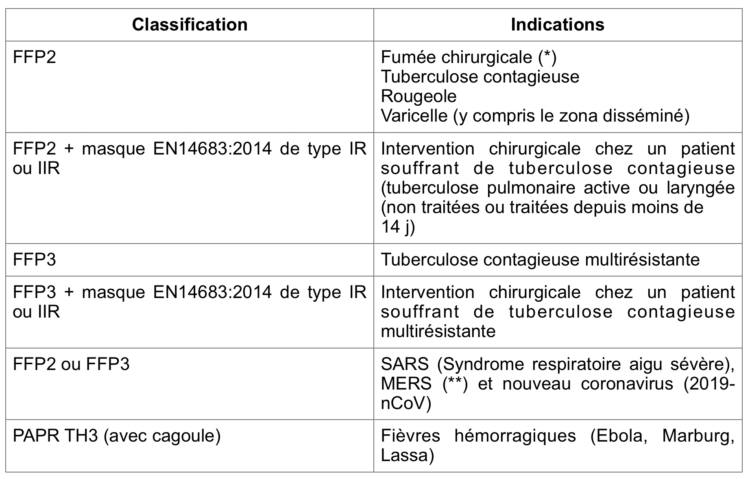
A. Gesser-Edelsburg, R. Cohen, M. Zemach, A. MirHalav.
Discourse on hygiene between hospitalized patients and health care workers as an accepted norm: Making it legitimate to remind health care workers about hand hygiene.
American Journal of Infection Control, Volume 48, Issue 1, January 2020, Pages 61-67
Background
Despite World Health Organization recommendations that patients should play a role in encouraging hand hygiene (HH) as a means of preventing infection, patient engagement remains an underused method. From the perspectives of hospitalized patients (HPs) and health care workers (HCWs) at 2 major public hospitals in Haifa, Israel, this research investigated (1) HP barriers to reminding HCWs to maintain HH, (2) HCW barriers to giving HPs instruction on proper hygiene, (3) what could help HPs and HCWs overcome these barriers, and (4) how video clips can be used to devise tailored strategies governing discourse on HH between HCWs and HPs.
Methods
Intervention type 2 design and examination of 2 population groups—HPs and HCWs—before and after intervention by means of mixed methods research.
Results
Both HPs and HCWs reported partial knowledge, embarrassment, and fears regarding commenting to staff, as well as a lack of cultural adaptation. The interviewees indicated that the video clips granted legitimacy to reminding HCWs about hygiene through strategies designed to identify and solve barriers, authenticity, and cultural adaptation.
Conclusions
To overcome HP and HCW barriers to maintaining HH, tailored video clips on HH should specify barriers and solutions with which they can both identify, thus turning discourse on HH into an accepted norm.
Jeanes P. G. Coen, N. S.Drey, D. J.Gould
Moving beyond hand hygiene monitoring as a marker of infection prevention performance: Development of a tailored infection control continuous quality improvement tool
American Journal of Infection Control, Volume 48, Issue 1, January 2020, Pages 68-76
Background
Infection control practice compliance is commonly monitored by measuring hand hygiene compliance. The limitations of this approach were recognized in 1 acute health care organization that led to the development of an Infection Control Continuous Quality Improvement tool.
Methods
The Pronovost cycle, Barriers and Mitigation tool, and Hexagon framework were used to review the existing monitoring system and develop a quality improvement data collection tool that considered the context of care delivery.
Results
Barriers and opportunities for improvement including ambiguity, consistency and feasibility of expectations, the environment, knowledge, and education were combined in a monitoring tool that was piloted and modified in response to feedback. Local adaptations enabled staff to prioritize and monitor issues important in their own workplace.
The tool replaced the previous system and was positively evaluated by auditors. Challenges included ensuring staff had time to train in use of the tool, time to collect the audit, and the reporting of low scores that conflicted with a target-based performance system.
Conclusions
Hand hygiene compliance monitoring alone misses other important aspects of infection control compliance. A continuous quality improvement tool was developed reflecting specific organizational needs that could be transferred or adapted to other organizations.
A. Benudis, S. Stone, A. Ssait, I. Mahoney, L. L. Price, A.Moreno-Koehler, E.Anketell, S.Doron
Pitfalls and Unexpected Benefits of an Electronic Hand Hygiene Monitoring System
American Journal of Infection Control, Volume 47, Issue 9, September 2019, Pages 1102-1106
Background
No single strategy is more effective than proper hand hygiene (HH) in reducing the spread of nosocomial infections. Unfortunately, health care worker compliance with HH is imperfect. We sought to improve HH compliance using an electronic hand hygiene monitoring system (EHHMS) in 2 units to collect unbiased data and provide feedback.
Methods
In this prospective, quasi-experimental study, the Hyginex EHHMS was installed in 2 units at Tufts Medical Center. Ninety-one bracelets were assigned, and electronic data were collected over 8 months. Human observations continued. We compared HH compliance as measured by human observation before, during, and after EHHMS implementation. Pre- and post-implementation surveys were distributed to staff.
Results
The number of electronically captured HH compliance observations was small due to infrequent bracelet use after month 2 of the intervention. HH compliance, as determined by human observation, increased by an average of 1.3 percentage points per month (P = .0005). Survey responses revealed negative attitudes about the EHHMS before and after its implementation.
Conclusions
Despite poor EHHMS participation and negative attitudes toward its implementation, HH compliance, as measured by human observation, significantly improved. Hospitals considering implementing an EHHMS should look to refine the intervention to encourage health care worker participation.
S. W. Bondurant, C. M.Duley, J. W.Harbell
Demonstrating the persistent antibacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application
American Journal of Infection Control, Volume 47, Issue 8, August 2019, Pages 928-932
Background
Use of hand sanitizers has become a cornerstone in clinical practice for the prevention of disease transmission between practitioners and patients. Traditionally, these preparations have relied on ethanol (60%-70%) for bactericidal action.
Methods
This study was conducted to measure the persistence of antibacterial activity of 2 preparations. One was a non-alcohol-based formulation using benzalkonium chloride (BK) (0.12%) and the other was an ethanol-based formulation (63%) (comparator product). The persistence of antibacterial activity was measured against Staphylococcus aureus using a technique modification prescribed in American Society for Testing and Materials protocol E2752-10 at up to 4 hours after application.
Results
The test product (BK) produced a marked reduction in colony-forming units at each of the 3 time points tested (3.75-4.16-log10 reductions), whereas the comparator produced less than 1-log10 reduction over the same time. The differences were highly significant.
Discussion
In the course of patient care or examination, there are instances where opportunities exist for the practitioner’s hands to become contaminated (eg, key boards and tables). Persistent antibacterial activity would reduce the chances of transfer to the patient.
Conclusions
These results show a major improvement in persistent antibacterial activity for the BK formulation compared to the comparator ethanol-based formulation.
M. P. Smiddy, O. M.Murphy, E. Savage, J. P. Browne
The influence of observational hand hygiene auditing on consultant doctors’ hand hygiene behaviors:
A qualitative study
American Journal of Infection Control, Volume 47, Issue 7, July 2019, Pages 798-803
Background
Compliance with hand hygiene guidelines reduces the risk of health care–associated infection, yet doctors are less compliant than other health care workers. Use of observational hand hygiene auditing with targeted individualized feedback was implemented, with improved hand hygiene of consultant doctors; however, the factors that influenced this were not explained by previous quantitative data. The aim was to explore consultant doctors’ opinions about the influence of observational hand hygiene auditing with individualized feedback on hand hygiene behavior.
Methods
Using the Theoretical Domains Framework, we conducted 12 semi-structured in-depth interviews with consultant doctors who experienced the observational hand hygiene audit and feedback intervention. Data were analyzed using a thematic analysis approach.
Results
Analysis identified 8 domains of the Theoretical Domains Framework, with 5 dominant domains: (1) behavioral regulation: receiving written individualized audit feedback positively influenced practice; (2) knowledge: provision of specific individualized feedback improved performance; (3) reinforcement: audit highlighted substandard practices; (4) social professional role and identity: audit reports triggered profession-associated competitive motivation; and (5) environmental context and resources: auditing was perceived to be synonymous with strong organizational safety culture.
Conclusions
In this study, provision of individualized targeted feedback was a critical component of observational hand hygiene auditing.
E. Evashwick, S. Cumplido, G. Eleby, T. Washington, S. Krishna, M. Almario, A. Hammonds-Reed, S. Fawcett, M. Ben-Aderet, J. Grein
A Tale of Two Departments: How Collaboration Between Infection Prevention and Sterile Processing Departments Can Improve Patient Safety
American Journal of Infection Control, Volume 47, Issue 6, Supplement, June 2019, Page S12
Background
Sterile Processing Departments (SPD) play a critical role in patient safety by proper sterilization of surgical instruments. Infection Preventionists (IPs) are often responsible for overseeing Infection Control (IC) standards for SPD, but frequently lack training in sterile processing that can create challenges. In 2015 we developed a quality improvement project improving IP knowledge of SPD processes and compliance with IC measures.
Methods
An audit tool was created utilizing observations, staff responses to IC questions and documentation review. To gain familiarity with SPD processes and improve audits, IPs attended weekly SPD meetings, Skills Fairs, APIC webinars and professional conferences . Audits were developed from nationally recommended practices, done bimonthly by IC personnel and reviewed quarterly with SPD leadership. IPs provided education for staff when gaps were identified. Implementation of SPD audits occurred concurrently with other OR quality improvement projects to facilitate improved instrument reprocessing
Results
Average overall audit scores increased in the first year from 67% in 2015 to 84% in 2016 (p < 0.001 by Chi squared test). Although overall average audit scores remained consistent since 2016, scores for questions related to employee knowledge of cleaning technique increased from 59% in 2015 to 90% in 2018 (p < 0.001). We observed a correlation between increasing SPD audit scores and decline in bioburden events from a yearly average of 3.29 events per 1000 procedures in 2015 to 1.15 events per 1000 procedures in 2018 (p < 0.001).
Conclusions
By attending SPD conferences, staff meetings, and skills fairs, IPs developed an audit tool to improve SPD oversight. Frequent auditing and education improved audit scores and correlated with an improvement in SPD function, as measured by decreased bioburden events. This project demonstrates how collaboration between the IP and SPD departments can lead to improved compliance and patient safety.
W. Kessler, T. Powers, FAPIC
Innovative Surveillance and Execution of the World Health Organization’s 5 Moments of Hand Hygiene in an Acute-care Hospital
American Journal of Infection Control, Volume 47, Issue 6, Supplement, June 2, 19, Page S23
Background
Hand hygiene (HH) is critical to the prevention of serious infections in the healthcare setting. Caregivers have been found to have suboptimal HH practices. The World Health Organization (WHO) 5 Moments of HH program was implemented in an acute-care hospital in an effort to increase caregiver participation, renew interest, and raise visibility.
Methods
Over a four month period in 2018, 128 participants were surveyed with an abbreviated WHO HH Perception Survey for Health-Care Workers in an acute-care and critical access hospital. Of the respondents, 67% were from nursing or ancillary nursing services. The abbreviated WHO HH Perception survey was utilized as a way to understand and improve caregiver perceptions and practices related to HH. A question highlighting HH improvement and the receipt of regular results regarding HH performance was targeted.
Results
On the WHO HH Self-Assessment Framework (SAF) the acute-care hospital scored 280 (range of 0 – 500), intermediate, for HH facility level and obtained a satisfactory level of 12 for HH Leadership. On a scale of 1 – 7, 73% of overall participants rated “receiving regular feedback regarding HH performance” ≥ 5 on the WHO HH perceptions survey. Based on this feedback and the WHO HHSAF, the acute-care hospital implemented a multimodal approach to HH practices through a novel framework.
Conclusions
This targeted approach illuminated participant values regarding regular feedback on HH performance. In response, infection prevention implemented secret shopper HH cameras that rotate departments in conjunction with frequent rounding on units to provide immediate feedback on HH compliance and missed opportunities. Feedback was provided to floor nurses, nurse managers, and assistant nurse managers. Additionally, a dynamic traveling HH campaign was implemented to socialize the WHO 5 Moments. The campaign asked all staff to participate in HH activities and to renew their commitment to HH through unit specific posters and signatures.
C. Mitchell, M. Ehly, C. Sacksteder, K. Zabriskie
Presentation Number ISR-76
Hand hygiene observations…not so secret anymore
American Journal of Infection Control, Volume 47, Issue 6, Supplement, June 2019, Page S29
Background
Tracking hand hygiene compliance (HHC) is an important function in healthcare to monitor compliance and validate accountability. Using a covert approach to monitoring hand hygiene limits the ability to collect a sufficient number of observations that are non-biased and highly reliable. Our objective is to create a program to reduce bias, be more reliable, yield a greater number of observations, and provide real time feedback.
Methods
We implemented an overt observation program to collect data and provide real-time feedback to staff at a 900-bed multi-facility academic medical center. Paid overt observers, who wear blue vests, used an electronic survey tool on mobile tablets to collect hand hygiene observations on all staff disciplines entering and exiting patient care areas. Observers had to collect both the entry and exit of the staff member to count as one observation. The observers also gave immediate feedback, both positive acknowledgment and constructive reminders for missed opportunities. Captured in real time, the data was distributed weekly and monthly to unit and discipline leadership.
Results
The total number of observations from the overt program was 24,598 for fiscal year (FY) 2016 compared to 8,548 covert observations in FY 2015. HHC improved from 68% to 88% from FY 2016 to FY 2017. The increase in HHC was statistically significant using the Chi square test. Verbal feedback given to staff increased from 67% in FY 2017 to 88% in FY 2018.
Conclusions
Since overt observers are not unit specific, they are able to provide an unbiased perspective when gathering observations. Assessments of the program are continuous. Observers complete competencies on an established schedule to ensure accuracy and consistency of each observer. With the implementation of the overt program, there was a statistically significant increase in the number of observations collected, improved compliance, and the ability to give and track feedback.
C. M. Hou, M. C.Rosenberg, R. A. Steer, C. Criccon, K. Campos
Presentation Number ISR-81
A Motion-Detection Electronic Hand Hygiene Verbal Reminder Increases Adherence in a Standard Precaution Room
American Journal of Infection Control, Volume 47, Issue 6, Supplement, June 2019, Page S31
Background
Hand hygiene rates remain suboptimal. We previously reported the effect of a motion-detection verbal hand hygiene reminder installed at the door of a contact isolation room and on hand hygiene for nurses, versus all others. This study evaluates the device’s effect on the hand hygiene rate for a standard precaution hospital room.
Methods
A wireless motion sensor was installed above a standard precaution room door. When an individual entered or exited the room, the device’s speaker announced a verbal hand hygiene reminder. The individual was credited with adherence by secret observers only if observed to wash hands at both entry and exit. This prospective study included 174 baseline measurements (before motion-detection sensor deployed) and 111 measurements during the intervention period (the period during which the sensor was installed), for a total of 285 observations.
Results
Before the wireless sensor was deployed, we found that without the verbal reminder, 6% (n=11) washed their hands at both entry and exit and 94% (n=163) did not. During the intervention, adherence increased to 38% (n=42), while 62% (n=69) did not. As different individuals might be observed before and after sensor installation, we performed a chi-square test for independence using Yates’ correction for continuity with SPSS 25 to compare rates of adherence. With the sensor, there was a statistically significant (X(squared)Yates(1) = 42.41, ϕ = .40, p<0.001) increase in hand hygiene adherence reflecting a nine-fold increase in the likelihood of compliance (odds ratio = 9.02, 95% Confidence Level 4.39-18.55)).
Conclusions
In this study, the use of a wireless motion sensor to provide verbal reminders significantly increased hand hygiene adherence at both entry and exit for a standard precaution room. Thus, an audio reminder triggered by motion detection can increase hand hygiene adherence and may curb the incidence of nosocomial infections.
E. Yoo, L. Ursua, R. Clark, J. Seok, J. Jeon, H. BinKim
The effect of incorporating covert observation into established overt observation-based hand hygiene promotion programs
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 482-486
Background
Covert observation (CO) is reliable for measuring hand hygiene compliance (HHC). However, the benefit of adding CO to overt observation (OO) is uncertain. We evaluated whether incorporating CO into an OO-based hand hygiene (HH) promotion program improves HH rate.
Methods
Health care worker’s HH activities were observed through 5 monitoring sessions (2 in phases 1 and 2 and 1 in phase 3) of simultaneous CO and OO. An intervention was applied—barrier identification interview—only in phase 2.
Results
Overall HHC was 91.0% for OO, and 49.3% for CO. HHC in phase 1 was not changed by repeated CO (34.7% and 34.0%, P = .70). HHC based on CO increased to 66.9% in phase 2 after the application of an intervention (P < .01), but decreased to 57.5% in phase 3 (P < .01). HHC based on OO increased significantly between only the first and second sessions in phase 2 (90.8% and 94.5%, respectively, P = .01).
Discussion
Although CO did not significantly change behavior, HHC with CO responded promptly to the application and cessation of a new intervention.
Conclusions
CO reflects HHC change more reliably than does OO. However, it is uncertain whether CO will improve HHC.
J. A.Woodard, S. Leekha, S. S.Jackson, K. A.Thom
Beyond entry and exit: Hand hygiene at the bedside
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 487-491
Background
We aimed to assess compliance, knowledge, and attitudes regarding the World Health Organization (WHO) 5 moments for hand hygiene (HH).
Methods
We assessed HH compliance from July-August 2016, using a modified WHO HH observation form. A 26-question survey was used to assess health care personnel (HCP) knowledge, opinions, and barriers to HH. A subgroup of HCPs participated in a 2-round focused survey to assign priority to the moments.
Results
Three hundred two HH opportunities were observed in 104 unique HCP-patient interactions. HH was performed at 106 (35%) opportunities, 37% (25 of 68) before touching a patient, 9% (6 of 70) before aseptic procedures, 5% (1 of 22) after body fluid exposure or risk, 63% (55 of 88) after touching a patient, and 35% (19 of 54) after touching patient surroundings. Two hundred eighteen HCPs completed the survey; 63 (29%) were familiar with the WHO 5 moments but only 13 (21%) were able to recall all 5 moments. In the focused surveys, 46% (6 of 13) ranked “before aseptic procedure” as the most important HH moment, and 86% (11 of 13) identified “after touching patient surroundings” as the least important.
Conclusions
We found frequent opportunities for HH with infrequent compliance. Lack of recognition of opportunities at the bedside and frequent glove use may contribute to lower compliance.
J. Baloh, K. A.Thom, E.Perencevich, C. Rock, G. Robinson, M. Ward, L. Herwaldt, H. S. Reisinger
Hand hygiene before donning nonsterile gloves: Healthcare workers’ beliefs and practices
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 492-497
Background
Understanding the perceptions and beliefs of health care workers (HCWs) regarding glove use and associated hand hygiene (HH) may be informative and ultimately improve practice. Research in this area is limited. This study examined the practices and beliefs of HCWs surrounding the use of nonsterile gloves and HH before gloving.
Methods
The study was conducted at 3 large academic US hospitals using a parallel convergent mixed-method design. To estimate compliance rates, the gloving and HH practices of HCWs were observed at entry to patient rooms for 6 months. Interviews were conducted with 25 providers, nurses, and nursing assistants to investigate their beliefs and perceptions of these practices.
Results
Observed HH compliance rates before gloving were 42%, yet in the interviews most HCWs reported 100% compliance. Observed compliance with gloving before entering contact precaution rooms was 78%, although all HCWs reported always gloving for standard and contact precautions. Most HCWs described using gloves more often than necessary. HCWs generally use gloves for their own safety and sanitize hands before gloving for patient safety. Numerous barriers to compliance with HH before gloving were discussed, including beliefs that gloves provide enough protection.
Conclusions
HH and glove use are highly intertwined in clinical practice and should be considered jointly in infection prevention improvement efforts.
E. T. Paul, M. Kuszajewski, A. Davenport, J. A.Thompson, B. Morgan
Sleep safe in clean hands: Improving hand hygiene compliance in the operating room through education and increased access to hand hygiene products
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 504-508
Background
Hand hygiene compliance is low among anesthesia providers in the operating room, which places patients at risk of preventable infections. The goal of this project was to improve hand hygiene compliance by educating anesthesia providers on the World Health Organization’s 5 indications for hand hygiene, and increasing access to hand hygiene products in the operating room.
Methods
Observations of hand hygiene in the operating room took place in 3 phases: preimplementation, postimplementation, and 60 days postimplementation.
Results
The results showed significant improvements in compliance for each of the 5 indications for hand hygiene as well as overall compliance. Each of the 3 phases of anesthesia demonstrated significant improvement as well. The results also showed a significant decrease in both glove use and use of the portable hand sanitizer device.
Discussion
Education and monitoring of hand hygiene among anesthesia providers in the operating room can improve hand hygiene compliance.
Conclusions
Although the use of the portable device declined, further studies could focus on observing single anesthesia providers instead of a preceptor/student combination, and also examine proximity to hand hygiene products in relation to compliance.
F. Sands, L. Fairbanks
How clean is “hygienically clean”: Quantitative microbial levels from samples of clean health care textiles across the United States
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 509-514
Background
In the United States, the laundry industry has not reliably measured microbial levels on hygienically clean textiles. The aim of this study was to quantitatively measure the microbial levels found on a sample of hygienically clean textiles.
Methods
Forty-eight health care textile samples were collected from hygienically clean linen scheduled to be used on 3 different patient care units. Samples were taken at 2 separate points in time representing laundry facility processing practices and hospital linen management practices. United States Pharmacopeia 61 testing was completed using a pour plate culturing method, producing a total aerobic microbial count and a total yeast and mold count.
Results
Of the samples, only 27% had a total aerobic microbial count below the expected 100 colony-forming unit level (range, 9-40,000) versus 81% (range, 9-1,000) for total yeast and mold count. Median microbial counts for the 2 separate time points across the 3 different patient care units were also higher than expected.
Conclusions
As far as we know, this study is a first step by the laundry industry to understand what quantitative microbial levels are currently found on hygienically clean health care textiles. These types of data can assist the industry in establishing appropriate outcome targets for process improvement initiatives.
M. M. Plemmons, J. Marcenaro, M. H.Oermann, J. Thompson, C. A.Vacchiano
Improving infection control practices of nurse anesthetists in the anesthesia workspace
American Journal of Infection Control, Volume 47, Issue 5, May 2019, Pages 551-557
Background
Anesthesia providers commonly cross-contaminate their workspace and subsequently put patients at risk for a health care–acquired infection. The primary objective of this project was to determine if education and implementation of standardized infection control guidelines that address evidence-based best practices would improve compliance with infection control procedures in the anesthesia workspace.
Methods
Patient care–related hand hygiene of nurse anesthetists was observed in 3 areas of anesthesia practice before and 3 weeks and 3 months after staff education, placement of visual reminders, and the implementation of infection control guidelines. After the observation periods, the percent compliance on the part of the providers was calculated for each of the 3 areas of anesthesia practice, and the results were compared using the Fisher exact test.
Results
There were a total of 95 observations performed during the 3 observation periods. When compared with preimplementation baseline data, there was a 26.2% increase in the number of providers compliant with hand hygiene practices after airway instrumentation (P = .029) and a 71.9% increase in the number of providers who separated clean from contaminated items in the workspace (P = .0001).
Conclusions
Education, visual reminders, and standardized infection control guidelines were shown to improve compliance with infection control best practices in a group of nurse anesthetists.
S. Pong, P. Holliday, G. Fernie
Effect of intermittent deployment of an electronic monitoring system on hand hygiene behaviors in healthcare workers
American Journal of Infection Control, Volume 47, Issue 4, April 2019, Pages 376-380
Background
Improving hand hygiene compliance among healthcare professionals is the most effective way to reduce healthcare–acquired infections. Electronic systems developed to increase hand hygiene performance show promise but might not maintain staff participation over time. In this study, we investigated an intermittent deployment strategy to overcome potentially declining participation levels.
Methods
An electronic monitoring system was deployed 3times at 6-month intervals on a musculoskeletal rehabilitation nursing unit in Toronto. Each deployment lasted 4 consecutive weeks. Each wall-mounted soap and hand rub dispenser was outfitted with an activation counter to assess the impact of system deployments on overall handwashing activity.
Results
System deployments took place in October 2016, April 2017, and October 2017. A total of 76,130 opportunities were recorded, with an aggregate hand hygiene performance of 67.43%. A total of 515,156 dispenser activations were recorded. There was a significant increase in aggregate dispenser use with every deployment and a decrease over several weeks following each withdrawal. Participation was high at the beginning of each deployment and declined during each deployment but was restored to a high level with the start of the next deployment.
Conclusions
Intermittent deployment of an electronic monitoring intervention counteracts potential declines in participation rates sometimes seen with continuous system use. However, adoption of this strategy requires the acceptance of lower periods of performance between each deployment.
M. Meng, M. Sorber, A. Herzog, C. IgelProf, C. Kugler
Technological innovations in infection control: A rapid review of the acceptance of behavior monitoring systems and their contribution to the improvement of hand hygiene
American Journal of Infection Control, Volume 47, Issue 4, April 2019, Pages 439-447
Background
Hand hygiene is crucial for preventing nosocomial infections; however, adherence rates need further attention. Prevention of nosocomial infections through regular hand hygiene monitoring and feedback is recommended by the World Health Organization. Technology holds the potential for achieving this goal. The aim of this study was to assess the influence of technological behavior monitoring innovations on hand hygiene adherence and their acceptance by healthcare professionals.
Methods
A rapid review of the literature was conducted. A literature search was performed in electronic databases (Cochrane Library, Scopus, PubMed, CINAHL, PsycINFO, PsycARTICLES, PSYNDEX) and via citation tracking in November 2017. Records were screened for eligibility. Included studies were analyzed and synthesized in a narrative, tabular way.
Results
Overall, 2,426 studies were identified, and 12 were included. Findings indicated that behavior monitoring technology improves hand hygiene adherence, resulting in adherence increases between 6.40%-54.97%. The majority of systems provided real-time feedback. Factors influencing acceptance of technology by healthcare professionals include transparency and confidentiality, user attitude and environment, device function, and device usability.
Conclusions
Recognizing the importance of hand hygiene adherence, active communication between behavior monitoring technology and healthcare workers seems to mediate improvement in sustainable hand hygiene adherence behavior.
A. Jeanes, P. G. Coen, D. J. Gould, N. S. Drey
Validity of hand hygiene compliance measurement by observation: A systematic review
American Journal of Infection Control, Volume 47, Issue 3, March 2019, Pages 313-322
Background
Hand hygiene is monitored by direct observation to improve practice, but this approach can potentially cause information, selection, and confounding bias, threatening the validity of findings. The aim of this study was to identify and describe the potential biases in hand hygiene compliance monitoring by direct observation; develop a typology of biases and propose improvements to reduce bias; and increase the validity of compliance measurements.
Methods
This systematic review of hospital-based intervention studies used direct observation to monitor health care workers’ hand hygiene compliance.
Results
Seventy-one publications were eligible for review. None was free of bias. Selection bias was present in all studies through lack of data collection on the weekends (n = 61, 86%) and at night (n = 46, 65%) and observations undertaken in single-specialty settings (n = 35, 49%). We observed inconsistency of terminology, definitions of hand hygiene opportunity, criteria, tools, and descriptions of the data collection. Frequency of observation, duration, or both were not described or were unclear in 58 (82%) publications. Observers were trained in 56 (79%) studies. Inter-rater reliability was measured in 26 (37%) studies.
Conclusions
Published research of hand hygiene compliance measured by direct observation lacks validity. Hand hygiene should be measured using methods that produce a valid indication of performance and quality. Standardization of methodology would expedite comparison of hand hygiene compliance between clinical settings and organizations.
S. Pong, P. Holliday, G. Fernie
Secondary measures of hand hygiene performance in health care available with continuous electronic monitoring of individuals
American Journal of Infection Control, Volume 47, Issue 1, January 2019, Pages 38-44
Background
Hand hygiene (HH) compliance in health care is usually measured against versions of the World Health Organization’s “Your 5 Moments” guidelines using direct observation. Such techniques result in small samples that are influenced by the presence of an observer. This study demonstrates that continuous electronic monitoring of individuals can overcome these limitations.
Methods
An electronic real-time prompting system collected HH data on a musculoskeletal rehabilitation unit for 12 weeks between October 2016 and October 2017. Aggregate and professional group scores and the distributions of individuals’ performance within groups were analyzed. Soiled utility room exits were monitored and compared with performance at patient rooms. Duration of patient room visits and the number of consecutive missed opportunities were calculated.
Results
Overall, 76,130 patient room and 1,448 soiled utility room HH opportunities were recorded from 98 health care professionals. Aggregate unit performance for patient and soiled utility rooms were both 67%, although individual compliance varied greatly. The number of hand wash events that occurred while inside patient rooms increased with longer visits, whereas HH performance at patient room exit decreased. Eighty-three percent of missed HH opportunities occurred as part of a series of missed events, not in isolation.
Conclusions
Continuous collection of HH data that includes temporal, spatial, and personnel details provides information on actual HH practices, whereas direct observation or dispenser counts show only aggregate trends.
M. Sande-Meijide, M. Lorenzo-González, F. Mori-Gamarra, I. Cortés-Gago, A. González-Vázquez, L. Moure-Rodríguez, M. Herranz-Urbasos
Perceptions and attitudes of patients and health care workers toward patient empowerment in promoting hand hygiene
American Journal of Infection Control, Volume 47, Issue 1, January 2019, Pages 45-50
Background
Patient empowerment is a component of the World Health Organization’s multimodal strategy to improve hand hygiene (HH). Its successful implementation requires knowledge of the perceptions and attitudes of patients and health care workers (HCWs) toward patient empowerment in HH.
Methods
A cross-sectional study, through a self-administered questionnaire of patients and their families and HCWs, was conducted in a 433-bed block of an 850-bed university hospital in Galicia, Spain.
Results
A total of 337 patients and their families and 196 HCWs completed the questionnaire. Among patients and their families, 49.9% were willing to remind HCWs about HH. However, only 31.6% of HCWs (41.8% of physicians and 24.8% of nurses) supported patient participation. The most common reason for patients and their families not being willing to ask caregivers to perform HH was fear of causing annoyance or receiving worse treatment as a consequence (76%). The main reasons that physicians disagreed with patient participation was patients’ lack of knowledge (40%) and possible negative effects on the HCW/patient relationship (40%). Nurses considered this participation unnecessary (58%).
Conclusions
There were significant differences between patients and their families and HCWs regarding support for patient empowerment in promoting HH. In our setting, a cultural change is needed in the HCW/patient relationship to create a facilitating environment.
J. Ho, S. H.Wong, V. C. Doddangoudar, M. V. Boost, G. Tse, M. Ip
Regional differences in temporal incidence of Clostridium difficile infection: a systematic review and meta-analysis
American Journal of Infection Control, January 2020, Volume 48, Issue 1 Pages 89-94
Background
Previous decades have witnessed a change in the epidemiology of Clostridium difficile infections. This study aimed to determine temporal trends in the incidence of C difficile infection across geographic regions.
Methods
An initial search of the relevant literature was conducted from date inception to October 2018 without language restriction. We estimated the pooled incidences using logit transformation, weighted by inverse variance. The Joinpoint Regression Analysis Program was used to explore its temporal trend.
Results
Globally, the estimated incidence of C difficile infection increased from 6.60 per 10,000 patient-days in 1997 to 13.8 per 10,000 patient-days in 2004. Thereafter, a significant downward trend was observed, at –8.75% annually until 2015. From 2005 to 2015, the incidences in most European countries decreased at a rate between 1.97% and 4.11% per annum, except in France, where an increasing incidence was observed (β = 0.16; P < .001). The incidences have stabilized in North America over the same period; however, in Asia, the incidence increased significantly from 2006 to 2014 (annualized percentage change = 14.4%; P < .001). The increase was greatest in Western Asian countries, including Turkey and Israel (β > 0.10; P < .004).
Conclusions
This study revealed rapid changes in the incidence of C difficile infection. This meta-analysis should inform the allocation of resources for controlling C difficile infection and future surveillance efforts in countries where epidemiologic information on C difficile infection remains sparse.
D. Champredon, K.ZhangBHS, M. Smieja, S. M.Moghadas
Clostridium difficile intervention timelines for diagnosis, isolation, and treatment
American Journal of Infection Control, Volume 47, Issue 11, November 2019, Pages 1370-1374
Background
Developing timelines of nosocomial Clostridium difficile infection (CDI) is critical to improving control and preventive measures. The objective of this study was to provide data-driven estimates of CDI timelines of diagnosis, isolation, and treatment in a hospital setting.
Methods
We obtained data for all CDI inpatients with symptoms onset occurring between January 1, 2013, and December 30, 2017, from St Joseph’s Healthcare in Hamilton, Canada. We analyzed full empirical distributions of timelines associated with the diagnosis, isolation, and treatment of CDI.
Results
A total of 683 inpatients with CDI symptoms were recorded, of which 243 cases were identified as health care–associated infection (HAI). The mean time intervals between the onset of CDI symptoms after admission and the release of laboratory results were 1.2 days and 1.9 days for the HAI and community-associated infection (CAI) patient groups, respectively. The mean time intervals from symptoms onset to the start of isolation were 1.5 days and 2.6 days for the corresponding patient groups. The initiation of treatment within 2 days of symptoms onset reduced the duration of first isolation (P value < .0001); however, the type of initial antibiotic used for CDI treatment was not associated with the duration of isolation.
Conclusions
Estimated timelines did not differ (P values > .6) between HAI and CAI patient groups with symptoms onset after admission. These estimates are useful for evaluating the effectiveness of CDI interventions.
K. McLaren, E. McCauley, B. O’Neill, S. Tinker, N. Jenkins, L. Sehulster
The efficacy of a simulated tunnel washer process on removal and destruction of Clostridioides difficile spores from health care textiles
American Journal of Infection Control, Volume 47, Issue 11, November 2019, Pages 1375-138
Background
Research on reducing Clostridioides difficile spore contamination of textiles via laundering is needed. We evaluated the sporicidal properties of 5 laundry chemicals and then determined the ability of a peracetic acid (PAA) laundry cycle to inactivate and/or remove spores from cotton swatches during a simulated tunnel washer (TW) process.
Methods
In phase I, spore-inoculated swatches were immersed in alkaline detergent, sodium hypochlorite, hydrogen peroxide, or PAA for 8 minutes. In phase II, inoculated swatches were passed through a simulated 24-minute TW process employing 5 wash liquids. Spore survivors on swatches and in test chemical fluids in both studies were enumerated using standard microbiologic assay methods.
Results
In phase I, hypochlorite solutions achieved >5 log10 spore reductions on swatches and >3 log10 reductions for wash solutions. PAA achieved minimal spore reduction in the wash solution (0.26 log10). In phase II, the PAA equilibrium-containing process achieved a >5 log10 spore reduction on swatches. In wash solution tests, the cumulative spore reduction peaked at >3.08 log10 in the final module.
Conclusions
Sodium hypochlorite as a laundry additive is sporicidal. The cumulative effects of a TW process, coupled with a PAA bleach agent at neutral pH, may render textiles essentially free of C difficile spore contamination.
E.Verheyen, V. Dalapathi, S. Arora, K. Patel, P. K. Mankal. V. Kumar, E. Lung, D.P. Kotler, A. Grinspan
High 30-day readmission rates associated with Clostridium difficile infection
American Journal of Infection Control, Volume 47, Issue 8, August 2019, Pages 922-927
Background
Clostridium difficile infection (CDI) is a leading cause of community-onset and healthcare–associated infection, with high recurrence rates, and associated high morbidity and mortality. We report national rates, leading causes, and predictors of hospital readmission for CDI.
Methods
Retrospective study of data from the 2013 Nationwide Readmissions Database of patients with a primary diagnosis of CDI and re-hospitalization within 30-days. A multivariate regression model was used to identify predictors of readmission.
Results
Of 38,409 patients admitted with a primary diagnosis of CDI, 21% were readmitted within 30-days, and 27% of those patients were readmitted with a primary diagnosis of CDI. Infections accounted for 47% of all readmissions. Female sex, anemia/coagulation defects, renal failure/electrolyte abnormalities and discharge to home (versus facility) were 12%, 13%, 15%, 36%, respectively, more likely to be readmitted with CDI.
Conclusions
We found that 1-in-5 patients hospitalized with CDI were readmitted to the hospital within 30-days. Infection comprised nearly half of these readmissions, with CDI being the most common etiology. Predictors of readmission with CDI include female sex, history of renal failure/electrolyte imbalances, anemia/coagulation defects, and being discharged home. CDI is associated with a high readmission risk, with evidence of several predictive risks for readmission.
V. R.Srinivasa, R. Hariri, L. R.Frank, L. Kingsley, E. Magee, M. Pokrywka, M. H.Yassin
Hospital-associated Clostridium difficile infection and reservoirs within the hospital environment
American Journal of Infection Control, Volume 47, Issue 7, July 2019, Pages 780-785
Background
Clostridium difficile infection (CDI) is a leading cause of hospital-associated infections. Antibiotic stewardship, environmental disinfection, and reduction of transmission via health care workers are the major modes of CDI prevention within hospitals.
Methods
The aim of this study was to evaluate the role of the environment in the spread of CDI within hospital rooms. Bed tracing of positive-CDI inpatients was performed to detect the strength of association to specific rooms. Environmental cultures were conducted to identify adequacy of environmental C difficile (CD) spores. Whole-genome sequencing was performed to evaluate the degree of CD relatedness.
Results
Bed tracing performed for 211 CDI patients showed a limited list of high-burden rooms. Environmental cultures for surfaces disinfected with a sporicidal agent were almost entirely negative, whereas the floors were positive for CDI in 15% of the studied patient rooms. Whole-genome sequencing did not detect any close genetic relatedness.
Conclusions
Unlike in an outbreak setting, bed tracing did not yield conclusive results of room reservoirs. The C diff Banana Broth culture was inexpensive, sensitive, and easy to incubate under aerobic conditions. Sporicidal disinfectants were effective in eliminating CD from the environment. CD spores were found on floors and hard-to-clean surfaces.
P. Sampathkumar, C. Folkert, J. E.Barth, L. Nation, M. Benz, A. Hesse, C. L.Mielke, K. W. Zaveleta
A trial of pulsed xenon ultraviolet disinfection to reduce Clostridioides difficile infection
American Journal of Infection Control, Volume 47, Issue 4, April 2019, Pages 406-408
Background
An intervention was designed to test whether the addition of an ultraviolet (UV) disinfection step after terminal cleaning would be helpful in reducing Clostridium difficile infection (CDI) rates in a real-world situation.
Methods
This study was a quasi-experimental design using 3 units as intervention units for the intervention and 3 similar units as control units. Intervention units 2 hematology and bone marrow transplant units and one medical-surgical unit at a large teaching hospital in the Midwest. UV disinfection was added after patient discharge and terminal cleaning in the intervention units.
Results
At baseline, CDI rates in the intervention and control arms were similar. During the 6 months of UV disinfection, the CDI rate in the intervention units decreased to 11.2 per 10,000 patient days, compared with 28.7 per 10,000 patient days in the control units (P = .03). In addition, the intervention units also saw a reduction in vancomycin-resistant enterococci acquisition.
Conclusions
The addition of UV disinfection to the terminal cleaning resulted in a reduction in CDI that has been sustained over several months 2 years.
C. S.Tilton, S. W. Johnson,
Development of a risk prediction model for hospital-onset Clostridium difficile infection in patients receiving systemic antibiotics
American Journal of Infection Control, Volume 47, Issue 3, March 2019, Pages 280-284
Background
Clostridium difficile infection (CDI) is recognized as a significant challenge in health care. Identification of high-risk individuals is essential for the development of CDI prevention strategies. The objective of this study was to develop an easily implementable risk prediction model for hospital-onset CDI in patients receiving systemic antimicrobials.
Methods
This retrospective, case-control, multicenter study included adult patients admitted to Novant Health Forsyth Medical Center and Novant Health Presbyterian Medical Center from July 1, 2015, to July 1, 2017, who received systemic antibiotics. Cases were subjects with hospital-onset CDI; controls were subjects without a CDI diagnosis. Cases were matched 1:1 with controls by admitted medical unit type. Variables significantly associated with CDI were incorporated into a multivariate analysis. A logistic regression model was used to formulate a point-based risk prediction model. Positive predictive value, negative predictive value, sensitivity, specificity, and accuracy were determined at various point cutoffs of the model. A receiver operating characteristic–area under the curve was created to assess the discrimination of the model.
Results
A total of 200 subjects (100 cases and 100 controls) were included. Most patients were Caucasian and female. Risk factors for CDI identified and incorporated into the model included age ≥70 years (adjusted odds ratio, 1.89; 95% confidence interval 1.05-3.43; P = .0326) and recent hospitalization in the past 90 days (adjusted odds ratio, 3.55; 95% confidence interval 1.90-6.83; P < .0001). Sensitivity and specificity were 76% and 49%, respectively, for scores ≥2 and 20% and 93%, respectively, for a score of 6. Diagnostic performance of various score cutoffs for the model indicated that a score ≥2 was associated with the highest accuracy (63%). The receiver operating characteristic–area under the curve was 0.7.
Discussion
We developed a simple-to-implement hospital-onset CDI risk model that included only independent risks that can be obtained immediately on presentation to the health care facility. Despite this, the model had fair discriminatory power. Similar risk factors were found in previously developed models; however, the utility of these models is limited owing to the difficulty of assessing other included risk factors and the inclusion of risk factors that cannot be evaluated until the patient is discharged from the health care facility.
Conclusions
Identification of hospitalized patients who are receiving systemic antibiotics, are ≥70 years old, and were recently admitted to the hospital in the past 90 days may allow for an easily implementable hospital-onset CDI risk prevention strategy.
K. A. Carter, A. N. Malani
Laxative use and testing for Clostridium difficile in hospitalized adults: An opportunity to improve diagnostic stewardship
American Journal of Infection Control, Volume 47, Issue 2, February 2019, Pages 170-174
Background
It is recommended that that only unformed stool from patients with diarrhea be tested for Clostridium difficile infection. We determined the prevalence of and patient characteristics associated with antecedent laxative receipt among hospitalized adults undergoing C difficile testing.
Methods
In a case-control study of 5,452 C difficile tests from 5 hospitals in Southeast Michigan, patients who received laxatives (docusate, senna, polyethylene glycol 3350, bisacodyl, and magnesium hydroxide) in the 24 or 48 hours before testing were identified. Logistic regression was performed to identify patient characteristics associated with laxative receipt before testing.
Results
In 535 (9.8%) and 707 (13%) tests, patients received laxatives in the 24 and 48 hours before testing, respectively. The odds of antecedent laxative receipt were significantly greater for patients residing on a surgical service than a medical service (24 hours odds ratio [OR], 2.5; 95% confidence interval [CI], 2.1–3.1; 48 hours OR, 2.7; 95% CI, 2.3–3.2), patients residing in an intensive care unit (ICU) than a non-ICU (24 hours OR, 1.3; 95% CI, 1.0–1.6; 48 hours OR, 1.3; 95% CI, 1.1–1.6), and patients whose Elixhauser Comorbidity Score was 4 or higher (24 hours OR, 1.4; 95% CI, 1.1–1.7; 48 hours OR, 1.4; 95% CI, 1.2–1.7).
Conclusions
Among patients tested for C difficile, antecedent laxative use was common. Improving diagnostic stewardship around C difficile testing, particularly in surgical and ICU patients, is a significant opportunity and priority for quality improvement.
M. L. Carvour, S. L. Wilder, K. L.Ryan, C. Walraven, F. Qeadan, M. Brett, K. Page
Predictors of Clostridium difficile infection and predictive impact of probiotic use in a diverse hospital-wide cohort
American Journal of Infection Control, Volume 47, Issue 1, January 2019, Pages 2-8
Background
Hospital-based predictive models for Clostridium difficile infection (CDI) may aid with surveillance efforts.
Methods
A retrospective cohort of adult hospitalized patients who were tested for CDI between May 1, 2011, and August 31, 2016, was formed. Proposed clinical and sociodemographic predictors of CDI were evaluated using multivariable predictive logistic regression modeling.
Results
In a cohort of 5,209 patients, including 1,092 CDI cases, emergency department location (adjusted odds ratio [aOR], 1.91; 95% confidence interval [CI], 1.51, 2.41; compared with an intensive care unit reference category, which had the lowest observed odds in the study) and prior exposure to a statin (aOR, 1.26, 95% CI, 1.06, 1.51), probiotic (aOR, 1.39; 95% CI, 1.08, 1.80), or high-risk antibiotic (aOR, 1.54; 95% CI, 1.29, 1.84), such as a cephalosporin, a quinolone, or clindamycin, were independent predictors of CDI. Probiotic use did not appear to attenuate the odds of CDI in patients exposed to high-risk antibiotics, but moderate-risk antibiotics appeared to significantly attenuate the odds of CDI in patients who received probiotics.
Conclusions
Emergency department location, high-risk antibiotics, probiotics, and statins were independently predictive of CDI. Further exploration of the relationship between probiotics and CDI, especially in diverse patient populations, is warranted.
G. DiDiodato, L. Fruchter
Antibiotic exposure and risk of community-associated Clostridium difficile infection: A self-controlled case series analysis
American Journal of Infection Control, Volume 47, Issue 1, January 2019, Pages 9-12
Background
Community-associated Clostridium difficile infection is inconsistently associated with antibiotic exposure. This study uses a self-controlled case series (SCCS) design to estimate antibiotic exposure effect sizes and compare them with those estimated from previous case-control studies.
Methods
Clostridium difficile infection among 139,000 patients registered to the Barrie Family Health Team from January 1, 2011, to May 1, 2017, using an SCCS design. Poisson regression analysis was used to estimate the incidence rate ratio (IRR) between antibiotic exposure versus nonexposure periods within individuals. Antibiotic exposure was categorized as either high risk (fluoroquinolone, clindamycin, or cephalosporin) or low risk (all other antibiotic classes).
Results
The final analysis included 189 cases. The pooled IRR for high-risk antibiotics was 2.26 (95% confidence interval [CI] 1.29, 3.98) and 2.03 (95% CI 1.19, 3.47) for lower-risk antibiotics. There was no difference between high-risk and lower-risk antibiotics (IRR 1.11, 95% CI 0.53, 2.36).
Interpretation
The IRRs were smaller than the odds ratios reported in previous case-control studies, suggesting a less biased estimate because SCCS designs control for time-invariant confounders. Compared with case-control studies, SCCS designs are underused in infection prevention and control studies.
Leitner E, Schreiner E, Neuhold M, Bozic M, Pux C, Pichler G, Schippinger W, Steinmetz I, Krause R, Zollner-Schwetz I.
Low prevalence of Clostridium difficile colonization in patients in long-term care facilities in Graz, Austria: A point-prevalence study.
Am J Infect Control. 2020 Jan 6.
Background
We aimed to determine the prevalence of asymptomatic colonization by C. difficile in stool of residents in four long-term care facilities (LTCFs) in Graz, Austria and to identify factors associated with colonization.
Methods
We conducted a point-prevalence study in March 2018. Stool samples were examined by GDH enzyme immunoassay and when positive a toxin A/B-enzyme immunoassay was carried out. Additionally, all samples were tested by toxin A and B PCR and were plated manually as well as in automated fashion onto selective C. difficile agar.
Results
In 4/144 (2.8%) residents the GDH assay was positive. Each resident was colonized by a different C. difficile ribotype. C. difficile was not detected in any of the environmental samples. Significantly more colonized residents (60%) had stayed at a hospital in the 3 months previous to the study compared to 10% of non-colonized patients (p=0.01).
Conclusions
The prevalence of colonization by toxigenic C. difficile was 2.8% in patients in LTCFs in Graz, Austria.
Copyright © 2019 Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved.
Marra AR, Perencevich EN, Nelson RE, Samore M, Khader K, Chiang HY, Chorazy ML, Herwaldt LA1, Diekema DJ, Kuxhausen MF, Blevins A, Ward MA, McDanel JS, Nair R, Balkenende E, Schweizer ML.
Incidence and Outcomes Associated With Clostridium difficile Infections: A Systematic Review and Meta-analysis.
JAMA Netw Open. 2020 Jan 3;3(1)
Importance
An understanding of the incidence and outcomes of Clostridium difficile infection (CDI) in the United States can inform investments in prevention and treatment interventions.
Objective
To quantify the incidence of CDI and its associated hospital length of stay (LOS) in the United States using a systematic literature review and meta-analysis.
Data Sources
MEDLINE via Ovid, Cochrane Library Databases via Wiley, Cumulative Index of Nursing and Allied Health Complete via EBSCO Information Services, Scopus, and Web of Science were searched for studies published in the United States between 2000 and 2019 that evaluated CDI and its associated LOS.
Study Selection
Incidence data were collected only from multicenter studies that had at least 5 sites. The LOS studies were included only if they assessed postinfection LOS or used methods accounting for time to infection using a multistate model or compared propensity score-matched patients with CDI with control patients without CDI. Long-term-care facility studies were excluded. Of the 119 full-text articles, 86 studies (72.3%) met the selection criteria.
Data Extraction and Synthesis
Two independent reviewers performed the data abstraction and quality assessment. Incidence data were pooled only when the denominators used the same units (eg, patient-days). These data were pooled by summing the number of hospital-onset CDI incident cases and the denominators across studies. Random-effects models were used to obtain pooled mean differences. Heterogeneity was assessed using the I2 value. Data analysis was performed in February 2019.
Main Outcomes and Measures
Incidence of CDI and CDI-associated hospital LOS in the United States.
Results
When the 13 studies that evaluated incidence data in patient-days due to hospital-onset CDI were pooled, the CDI incidence rate was 8.3 cases per 10 000 patient-days. Among propensity score-matched studies (16 of 20 studies), the CDI-associated mean difference in LOS (in days) between patients with and without CDI varied from 3.0 days (95% CI, 1.44-4.63 days) to 21.6 days (95% CI, 19.29-23.90 days).
Conclusions and Relevance
Pooled estimates from currently available literature suggest that CDI is associated with a large burden on the health care system. However, these estimates should be interpreted with caution because higher-quality studies should be completed to guide future evaluations of CDI prevention and treatment interventions.
Kachrimanidou M, Tzika E, Filioussis G.
Clostridioides (Clostridium) Difficile in Food-Producing Animals, Horses and Household Pets: A Comprehensive Review.
Microorganisms. 2019 Dec 9;7(12).
Abstract
Clostridioides (Clostridium) difficile is ubiquitous in the environment and is also considered as a bacterium of great importance in diarrhea-associated disease for humans and different animal species. Food animals and household pets are frequently found positive for toxigenic C. difficile without exposing clinical signs of infection. Humans and animals share common C. difficile ribotypes (RTs) suggesting potential zoonotic transmission. However, the role of animals for the development of human infection due to C. difficile remains unclear. One major public health issue is the existence of asymptomatic animals that carry and shed the bacterium to the environment, and infect individuals or populations, directly or through the food chain. C. difficile ribotype 078 is frequently isolated from food animals and household pets as well as from their environment. Nevertheless, direct evidence for the transmission of this particular ribotype from animals to humans has never been established. This review will summarize the current available data on epidemiology, clinical presentations, risk factors and laboratory diagnosis of C. difficile infection in food animals and household pets, outline potential prevention and control strategies, and also describe the current evidence towards a zoonotic potential of C. difficile infection.
Goltsman G, Gal G, Mizrahi EH, Mardanov S, Pinco E, Lubart E.
The impact of intensive staff education on rate of Clostridium difficile-associated disease in hospitalized geriatric patients.
Aging Clin Exp Res. 2019 Nov 27.
Background
Toxin-producing Clostridium difficile is the most common cause of nosocomial diarrhea in geriatric units.
Aim
The purpose of study was to check the impact of intensive staff education on rate of Clostridium difficile-associated disease in hospitalized geriatric patients.
Methods
The sampling frame was all patients suffering from diarrhea checked for Clostridium difficile toxin during the years 2017-2018. Clostridium difficile-positive patients were compared to a similar number of Clostridium difficile toxin-negative patients. The data were compared to our previous study, followed by medical staff’s educational program for Clostridium difficile control and prevention.
Results
Among 217 patients with diarrhea, 60 (27.6%) were positive for Clostridium difficile toxin. The study group tended to be of older age (p = 0.06), and showed higher rate of functional impairment (p < 0.001) and mortality (p < 0.001) than Clostridium difficile toxin negative patients. The rate of Clostridium difficile toxin-positive patients did not significantly differ between the previous and current studies (20.0% and 27.6%, respectively).
Conclusions and discussion
In spite of findings, that patients tended to be older, with high rate of mortality, the rate of Clostridium difficile did not change from the previous study.
Haigh J, Cutino-Moguel MT, Wilks M, Welch CA, Melzer M.
A service evaluation of simultaneous near-patient testing for influenza, respiratory syncytial virus, Clostridium difficile and norovirus in a UK district general hospital.
J Hosp Infect. 2019 Dec;103(4):441-446.
Background
The Cepheid® GeneXpert® (GXP) can simultaneously test for norovirus (NV), Clostridium difficile (CD), influenza A/B (IFA/B) and respiratory syncytial virus (RSV).
Aim
To compare centralized multiplex polymerase chain reaction (PCR) testing with localized GXP testing at a district general hospital.
Methods
From December 2017 to December 2018, samples received at Whipps Cross University Hospital (WCUH) were first tested at the local laboratory before transport centrally to the Royal London Hospital (RLH). At the RLH, a non-proprietary multiplex reverse transcriptase (RT) PCR assay was performed, which also tested for gastrointestinal or respiratory pathogens not tested for by the GXP.
Findings
A total of 1111 stool and respiratory samples were processed at both sites; 591 were respiratory and 520 were stool samples. Compared to centralized testing, the GXP gave sensitivity, specificity, and NPV all in excess of 97%, with the exception of RSV. The RSV assay had a sensitivity of 66.7% (95% confidence interval (CI) 24.1, 94.0) but an NPV of 99.7% (95% CI 98.6, 99.9). At the RLH, 65 (5.9%) additional respiratory or gastrointestinal viruses were detected, predominantly rhinovirus 35 (3.2%) and adenovirus 11 (1.0%). Compared to centralized testing, the median time saved for local respiratory and gastrointestinal sample testing was 19 h and 46 min and 17 h and 6 min, respectively.
Conclusions
Local GXP testing compared to centralized multiplex PCR testing for IF, NV and CD, demonstrated sensitivities, specificities and NPV between 95% and 100%. Turnaround times were faster, enabling quicker infection prevention and control decision making. In our local setting (WCUH), the GXP demonstrated the potential to reduce NV and IFA/B outbreaks.
Grainger RJ, Stevens NT, Humphreys H.
Approaches to the detection of Clostridioides difficile in the healthcare environment.J
Hosp Infect. 2019 Dec;103(4):375-381.
Abstract
Clostridioides difficile, a spore-forming bacillus, is a major cause of healthcare-associated infection, and can survive for prolonged periods in the inanimate environment. Environmental sampling to detect C. difficile is not routine but may be undertaken as part of outbreak management and during research projects. We conducted a literature search covering the period between 1980 and 2018 to review methods for the detection of this pathogen in the environment. There are many acceptable sampling methods used for environmental screening, including contact plates, cotton swabs, flocked swabs and sponges. Most recent studies suggest that sponges are the most effective method of sampling and have the added benefit of being capable of sampling larger and curved areas. Culture methods are the most common laboratory method of detecting C. difficile from environmental samples. However, the results are variable depending on the type of agar used and the turnaround times can be long. Molecular methods such as real-time polymerase chain reaction (RT-PCR), although more commonly used to detect C. difficile from faecal specimens, has been used with varying degrees of success in environmental sampling. Further studies are needed to determine whether molecular techniques could offer a more reliable, faster method of environmental sampling, giving infection prevention and control teams more reassurance that patients are being placed in adequately decontaminated hospital environments.